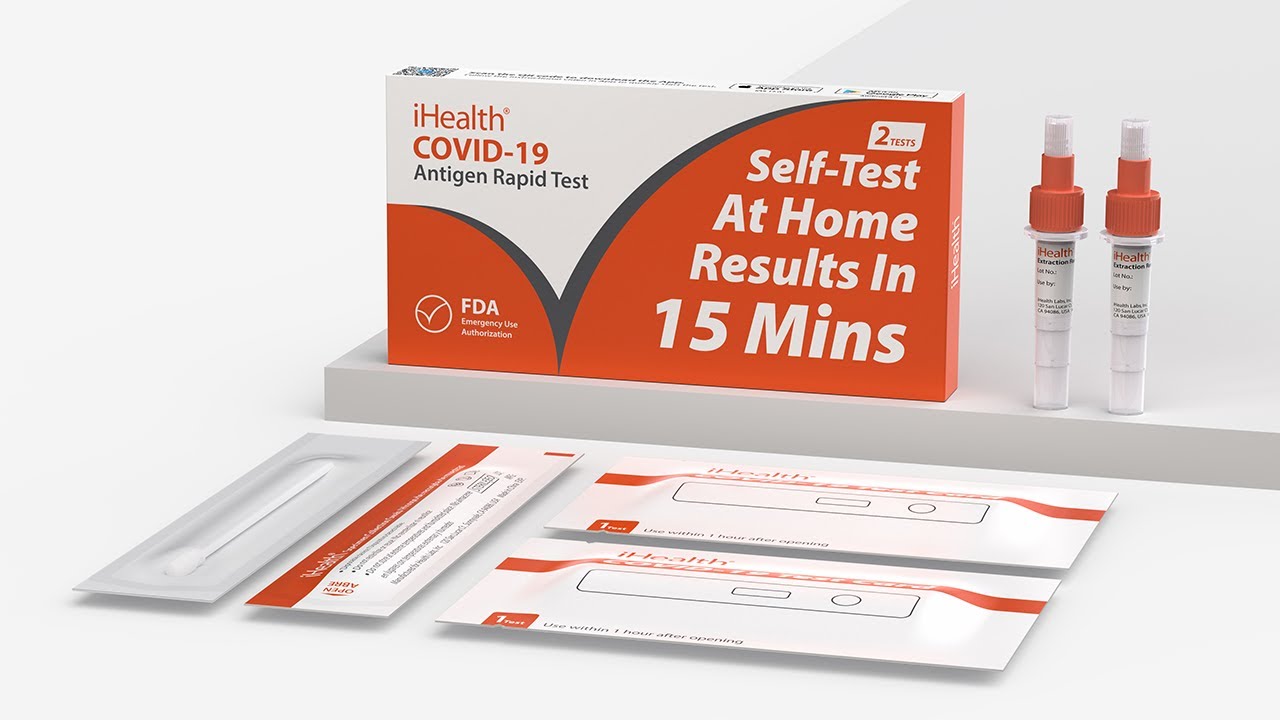
RAPID DIAGNOSTIC TEST FOR THE DETECTION OF SARS-COV-2 ANTIGEN
iHealth® Rapid Antigen Test
Self-Test at Home
Results in minutes.

iHealth® RAPID ANTIGEN TEST FOR
COVID-19

iHealth® COVID-19 Antigen Test
The iHealth® COVID-19 Antigen Rapid Test is a lateral flow assay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2.
This test is authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 15 years or older with symptoms of COVID-19 within the first 7 days of symptom onset. This test is also authorized for non-prescription home use with adult-collected nasal swab samples from individuals aged 2 years or older with symptoms of COVID-19 within the first 7 days of symptom onset.
This test is also authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 15 years or older, or adult collected anterior nasal swab samples from individuals aged 2 years or older, with or without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over three days with at least 24 hours (and no more than 48 hours) between tests.
RAPID DIAGNOSTIC TEST FOR THE DETECTION OF SARS-COV-2 ANTIGEN iHealth® Rapid Antigen Home Test – 2ct. Two (2) individual test per box. Rapid point-of-care (POC) test with results in minutes
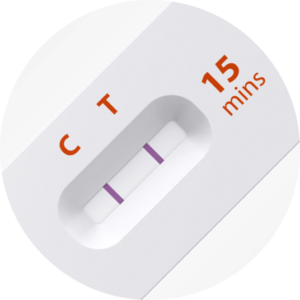
iHealth® COVID-19 ANTIGEN RAPID TEST DEVICE DEMONSTRATION
Resources
PRODUCT INFORMATION
iHealth® COVID-19 Antigen Rapid Test Product Information
FDA EMERGENCY USE AUTHORIZATION
FDA Authorizes Made in the USA Point-of-Care Antigen Test for COVID-19